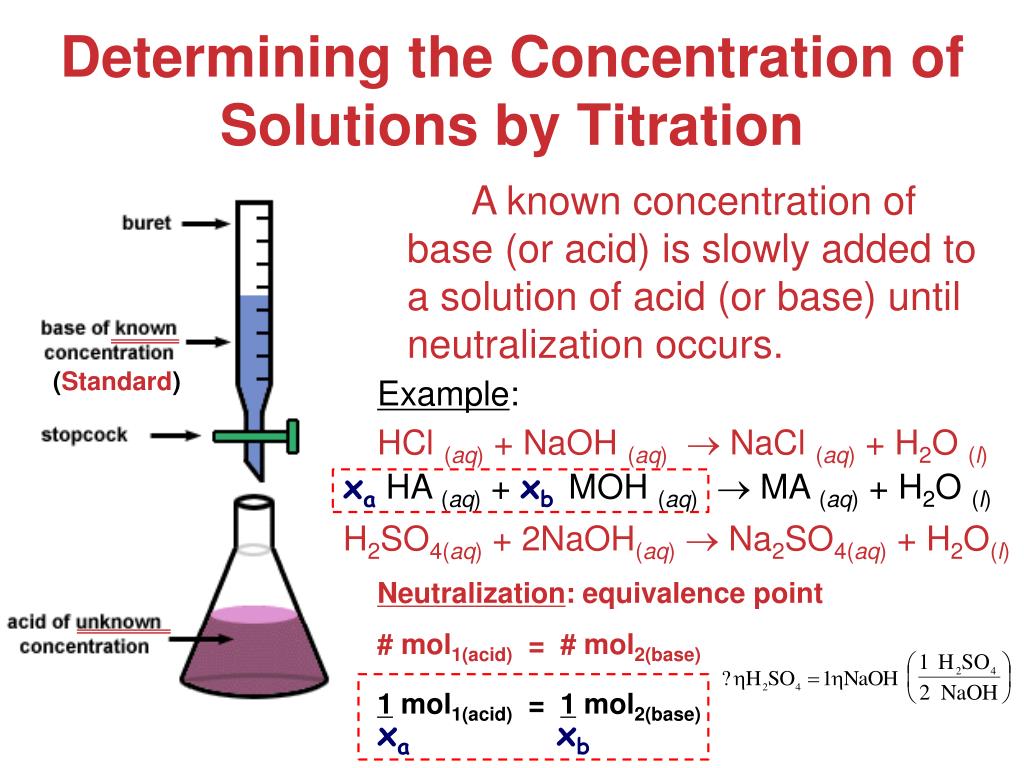
Titration Calculations Normality . Titration is the process of gradual addition of a solution of a known concentration and volume. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculation of normality in titration. calculation of normality in titration. Titration is the process of gradually adding a solution of a certain volume and concentration to another. calculation of normality in titration. the normality of a solution is the gram equivalent weight of a solute per liter of solution. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of.
from www.slideserve.com
a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. the normality of a solution is the gram equivalent weight of a solute per liter of solution. calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculation of normality in titration. Titration is the process of gradual addition of a solution of a known concentration and volume. Titration is the process of gradually adding a solution of a certain volume and concentration to another. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. calculation of normality in titration.
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
Titration Calculations Normality the normality of a solution is the gram equivalent weight of a solute per liter of solution. Titration is the process of gradual addition of a solution of a known concentration and volume. calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradually adding a solution of a certain volume and concentration to another. the normality of a solution is the gram equivalent weight of a solute per liter of solution. calculation of normality in titration. calculation of normality in titration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. calculation of normality in titration. Titration is the process of gradually adding a solution of a certain volume and concentration to another. the normality of a solution is the gram equivalent weight of a solute per liter of. Titration Calculations Normality.
From www.youtube.com
Titration Calculation (Example) YouTube Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradual addition of a solution of a known concentration and volume.. Titration Calculations Normality.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Calculations Normality calculation of normality in titration. the normality of a solution is the gram equivalent weight of a solute per liter of solution. Titration is the process of gradually adding a solution of a certain volume and concentration to another. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Calculations Normality.
From www.youtube.com
Normality Factor 08 Titration Class 11th & 12th YouTube Titration Calculations Normality the normality of a solution is the gram equivalent weight of a solute per liter of solution. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Titration Calculations Normality.
From www.science-revision.co.uk
Titrations Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume. calculation of normality in titration. calculation of normality in titration. Titration is the process of gradually adding a solution of a certain volume and concentration to another. At the equivalence point in a neutralization, the moles of acid are equal to the. Titration Calculations Normality.
From beingchemist.com
Titration Calculations and Formulas Being Chemist Titration Calculations Normality a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradually adding a solution of a certain volume. Titration Calculations Normality.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Calculations Normality At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradually adding a solution of a certain volume and concentration to another. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. the normality of a. Titration Calculations Normality.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Calculations Normality calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradually adding a solution of a certain volume and concentration to another. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Titration Calculations Normality.
From relationshipbetween.com
Difference Between Normality Factor And Titration Error Relationship Titration Calculations Normality the normality of a solution is the gram equivalent weight of a solute per liter of solution. calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration Calculations Normality.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. calculation of normality in titration. the normality of a. Titration Calculations Normality.
From www.slideserve.com
PPT Chapter 16 Solutions PowerPoint Presentation, free download ID Titration Calculations Normality Titration is the process of gradually adding a solution of a certain volume and concentration to another. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. calculation of normality in titration. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution. Titration Calculations Normality.
From www.slideserve.com
PPT Lewis Bases, Acid Base Titration, and Normality PowerPoint Titration Calculations Normality Titration is the process of gradually adding a solution of a certain volume and concentration to another. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. calculation of normality in. Titration Calculations Normality.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Calculations Normality calculation of normality in titration. the normality of a solution is the gram equivalent weight of a solute per liter of solution. Titration is the process of gradual addition of a solution of a known concentration and volume. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Titration is. Titration Calculations Normality.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Normality a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Titration is the process of gradually adding a solution of a certain volume and concentration to another. the normality of a solution is the gram equivalent weight of a solute. Titration Calculations Normality.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume with another solution of. calculation of normality in titration. the normality of a solution is the gram equivalent weight of a solute per liter of solution. calculation of normality in titration. a titration is a volumetric technique in which a. Titration Calculations Normality.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Calculations Normality Titration is the process of gradually adding a solution of a certain volume and concentration to another. Titration is the process of gradual addition of a solution of a known concentration and volume. calculation of normality in titration. calculation of normality in titration. calculation of normality in titration. Titration is the process of gradual addition of a. Titration Calculations Normality.
From www.youtube.com
15.4 Titrations & Normality YouTube Titration Calculations Normality calculation of normality in titration. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. the normality of a solution is. Titration Calculations Normality.
From learningschoolregibanb.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Calculations Normality Titration is the process of gradual addition of a solution of a known concentration and volume. calculation of normality in titration. the normality of a solution is the gram equivalent weight of a solute per liter of solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Titration Calculations Normality.